Aktinium
89
Ac
Groep
n/a
Periode
7
Blok
f
Protrone
Elektrone
Neutrone
89
89
138
Algemene Eienskappe
Atoom Nommer
89
Atoommassa
[227]
Massa Nommer
227
Kategorie
Aktiniede
Kleur
Silver
Radioaktief
Ja
From the Greek aktis, aktinos, meaning beam or ray
Kristalstruktuur
Gesiggesentreerde kubieke
Geskiendenis
André-Louis Debierne, a French chemist, discovered actinium in 1899.
He separated it from pitchblende residues left by Marie and Pierre Curie after they had extracted radium.
Friedrich Oskar Giesel independently discovered actinium in 1902 as a substance being similar to lanthanum.
He separated it from pitchblende residues left by Marie and Pierre Curie after they had extracted radium.
Friedrich Oskar Giesel independently discovered actinium in 1902 as a substance being similar to lanthanum.
Elektrone per skil
2, 8, 18, 32, 18, 9, 2
Elektronkonfigurasie
[Rn] 6d1 7s2
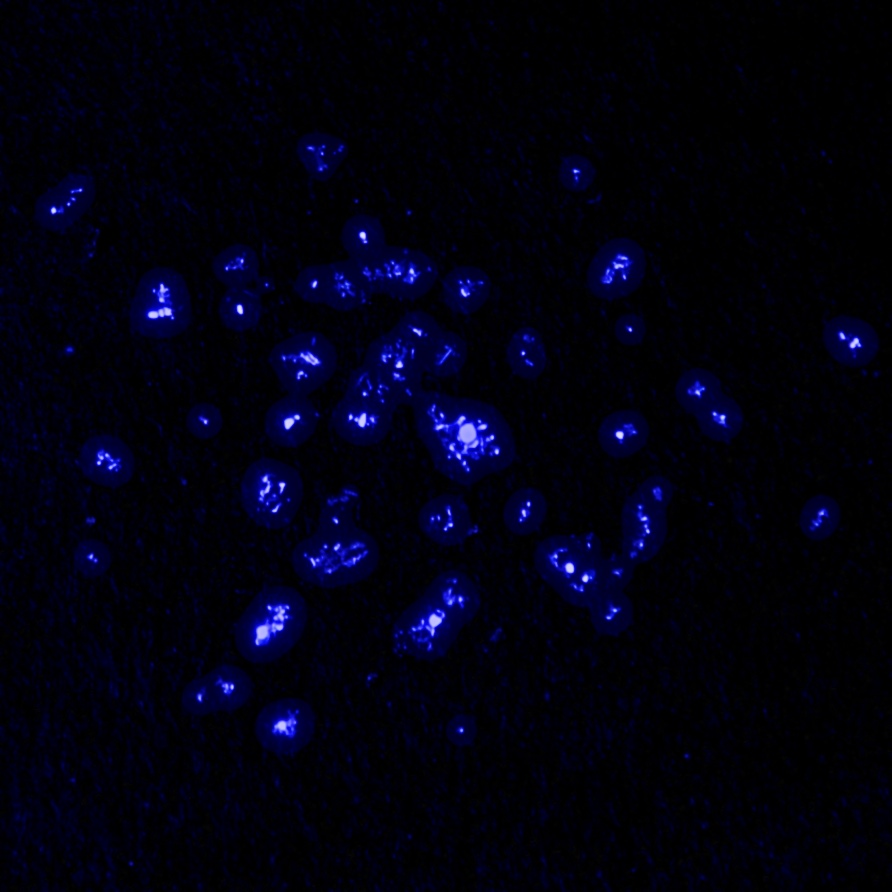
Actinium glows in the dark with a pale blue light
Fisiese Eienskappe
Fase
Soliede
Digtheid
10,07 g/cm3
Smeltpunt
1323,15 K | 1050 °C | 1922 °F
Kookpunt
3471,15 K | 3198 °C | 5788,4 °F
Heat of Fusion
14 kJ/mol
Heat of Vaporization
400 kJ/mol
Spesifieke Hitte Kapasiteit
0,12 J/g·K
Oorvloed in die aardkors
n/a
Oorvloed in die heelal
n/a
CAS Nommer
7440-34-8
PubChem CID Nommer
n/a
Atomiese Eienskappe
Atoom radius
-
Kovalente Radius
215 pm
Elektronegatiewiteit
1,1 (Pauling scale)
Ionisasie potensiaal
5,17 eV
Atoom volume
22,54 cm3/mol
Termiese geleidingsvermoë
0,12 W/cm·K
Oksidasiestate
3
Toepassings
Actinium is used as an active element of radioisotope thermoelectric generators, for example in spacecraft.
The medium half-life of 227Ac makes it very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters.
225Ac is applied in medicine to produce 213Bi in a reusable generator or can be used alone as an agent for radiation therapy.
The medium half-life of 227Ac makes it very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters.
225Ac is applied in medicine to produce 213Bi in a reusable generator or can be used alone as an agent for radiation therapy.
Actinium is highly radioactive
Isotope
Stabiele Isotope
-Onstabiele Isotope
206Ac, 207Ac, 208Ac, 209Ac, 210Ac, 211Ac, 212Ac, 213Ac, 214Ac, 215Ac, 216Ac, 217Ac, 218Ac, 219Ac, 220Ac, 221Ac, 222Ac, 223Ac, 224Ac, 225Ac, 226Ac, 227Ac, 228Ac, 229Ac, 230Ac, 231Ac, 232Ac, 233Ac, 234Ac, 235Ac, 236Ac